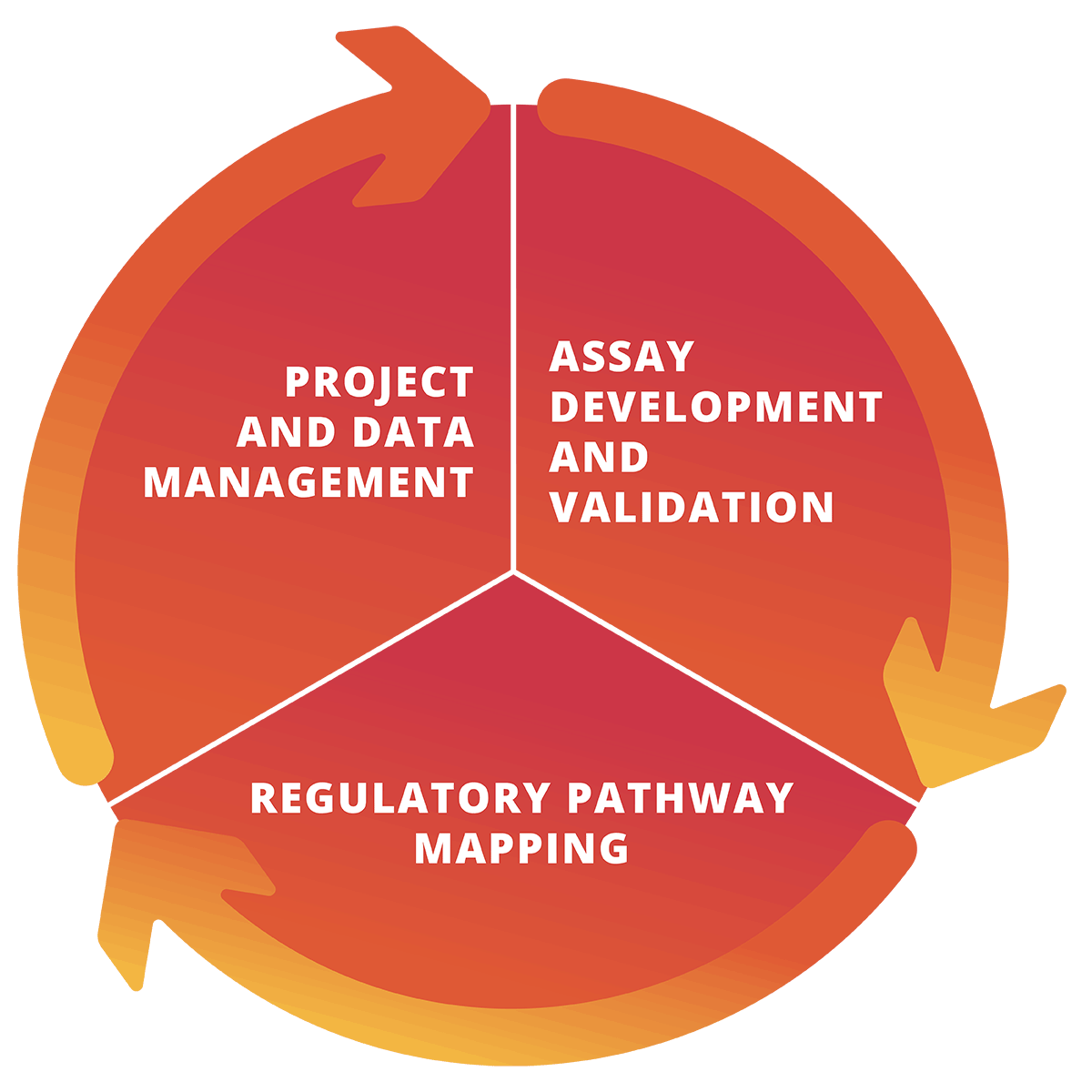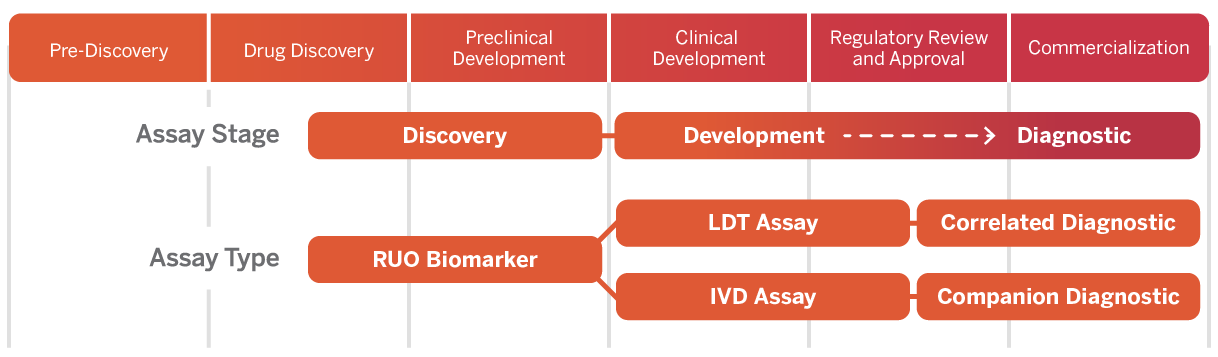
Laboratory & Diagnostic Services
Versiti Clinical Trial Services provides central laboratory services, including sample testing, assay development, site management and more.
HLA Testing & Cell Transplantation
Trust Versiti’s HLA Testing team for high-throughput, high-resolution typing solutions that support cell therapies and clinical development.
Molecular Diagnostics and Infectious Disease Testing
Versiti delivers precision Molecular Diagnostics and Infectious Disease Testing backed by regulatory expertise and high-throughput capacity.